The following is adapted from a presentation that Bryan Oronsky and Scott Caroen from the pharmaceutical company, EpicentRx, gave to the OneTri Conference over Zoom in Brisbane, Australia on October 3, 2023.

The Imitation Game
The tagline from a commercial by Doritos in the 1980’s for their flavored tortilla chips that featured US talk show host and comedian, Jay Leno, as pitchman was “Crunch all you want, we’ll make more.” The pharmaceutical industry added their own spin to this tagline, which is, “Take all you want, we’ll make more”. Another variation is “Take all you want, we’ll patent more”.
Drug development is not for the faint of heart. It takes 10-15 years to bring a new drug to market with an average cost of $1-2 billion. Add to this that over 90% of drugs in development fail and it is not hard to see why many pharmaceutical companies prefer to modify or upgrade what is already there rather than to innovate out of thin air. It takes nerve and verve to set a new course and direction, much simpler, safer, and cheaper is to follow behind on a path that has already been cleared of obstacles, make a few usually minor improvements or tweaks along the way—and then patent them.
This largely explains the marketed glut of patented, non-breakthrough drugs a.k.a. “me-too” or “mostly me-too drugs” that address an already satisfied need. Examples include beta-blockers for heart disease, H2-blockers and proton pump inhibitors for peptic ulcers and acid reflux disease, HMG-COA reductase inhibitors (statins) for hypercholesteremia, the SSRI and SNRI classes of antidepressants, opiates for pain, checkpoint inhibitors for treatment of cancer, and GLP-1 agonists for diabetes and weight loss, as shown in the “Drug Wheel” figure below. Meanwhile, many different disorders lack effective therapies and options for patients with them are few and far between.
Figure 1: The Drug Wheel
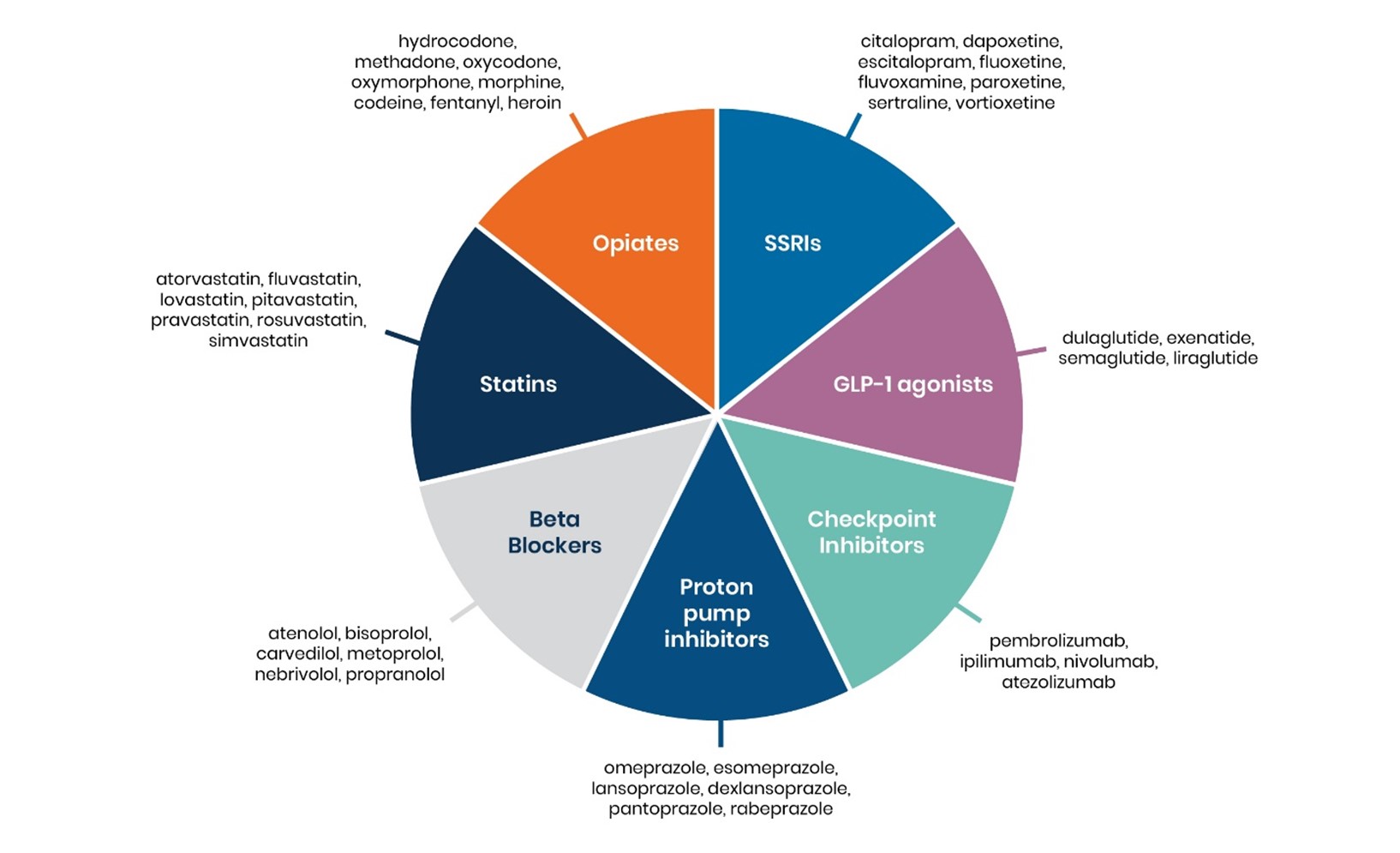
Why reinvent the wheel each time when pharmaceutical companies can simply pump up the tires, change the spokes and call it new?
Liftoff
Not at all content to play this imitation game was an uber-successful Silicon Valley-based venture capitalist and long-time Big Pharma executive—call him AO. According to AO, drug development was the very definition of insanity—because it followed the same old, same old time and time again and continued to expect different results.
Having decided to flip the imitative, cliched drug development script on its head, AO put his considerable resources behind a crack team that eventually became EpicentRx, a California biopharmaceutical company. Their mandate was to form a partnership with the American aerospace and defense industry (ADI), which they did. The intent of this partnership was to screen several high-energy rocket propellants for anticancer activity and to create truly novel therapeutic categories in the process. The expectation was that these propellants would decompose to form tumor-damaging free radicals in vivo, and free radicals synergize well with radiation, for example. However, two problems immediately presented themselves. The first was that nitrogen-rich rocket propellants are explosive. The second was that nitrogen-rich rocket propellants are explosive. Fortunately, hundreds and hundreds of them were available to screen. The tagline of this EpicentRx-ADI partnership could have been, “Explode all you want. We’ll make more”.
Still, no surprise, it turned out that the word “explosive” was not a big selling point with investors—or with the FDA. The solution was to replace one or more of the explosive side groups on these compounds with non-explosive ones. In the case of the small molecule that came to be known as RRx-001 (generic name nibrozetone) a nitro group on trinitroazetidine (TNAZ) was replaced with an acyl bromide group, as shown below, which rendered it non-explosive.
Figure 2: The Transformation of TNAZ to RRx-001
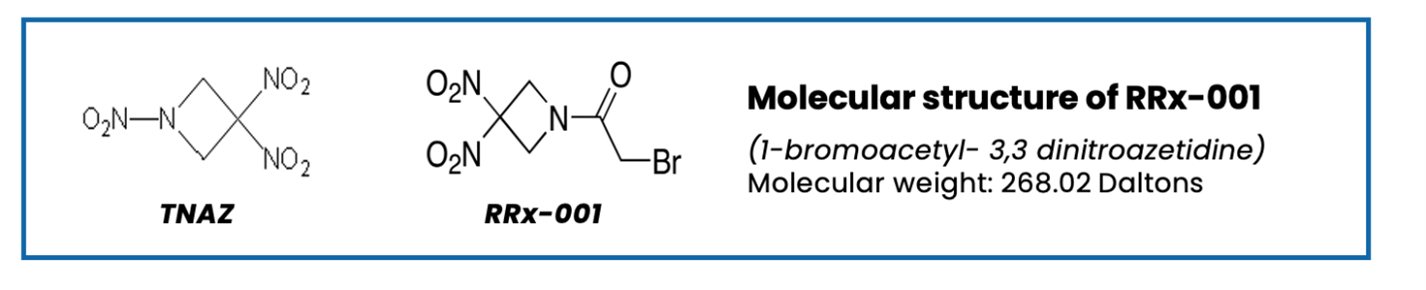
And just like that from nothing more than a germ of an idea EpicentRx (formerly known as RadioRx) achieved liftoff.
Cosmic Laughter
According to an old proverb, if you want God to laugh, tell Him your plans. Ditto the Drug Development Gods. EpicentRx announced that it planned to develop RRx-001/nibrozetone as a radiosensitizer, meaning a drug that partnered with radiation therapy and made it better.
All well and good because as it turned out RRx-001/nibrozetone was a radiosensitizer but also much, much more. Which presented a problem of sorts. Because small pharmaceutical companies like EpicentRx are funded to develop one molecule in one indication, not one molecule in several. Already RRx-001/nibrozetone was under investigation in two late-stage clinical trials—the first called REPLATINUM evaluates the combination RRx-001/nibrozetone plus a platinum chemotherapy doublet in Phase 3 for the treatment of 3rd line or beyond small cell lung cancer (SCLC), the second called KEVLAR combines RRx-001/nibrozetone with cisplatin and radiotherapy in 1st line head and neck cancer. And so, the company didn’t have the resources or the bandwidth to expand beyond those two clinical trials. Fortunately, however, EpicentRx managed to find investors in addition to AO that threw their financial support and their reputations behind the multi-indication potential of RRx-001/nibrozetone. Accordingly, the company expanded almost overnight from 5 full time employees to 15.
Fast forwarding ahead, one of those indications was neuroinflammation and neurodegenerative diseases like Parkinson’s, ALS/MND, and Alzheimer’s. However, EpicentRx was a company with anticancer not neurodegenerative expertise. Its CEO, Dr. Tony Reid, is a Stanford University-trained 25+ years practicing gastrointestinal (GI) oncologist and virologist who brought a platform of armed oncolytic adenoviruses with him when he came to the company in 2015. One of those oncolytic adenoviruses called AdAPT-001 that carries and expresses a TGF-β trap is in Phase 2 clinical trials for the treatment of cancer. Nevertheless, for all their experience in and with cancer, Tony Reid, and the rest of the EpicentRx team were out of their depth when it came to the treatment of neurodegenerative diseases. EpicentRx needed a partner.
A Meet QUT
Enter Dr. Richard Gordon, associate professor from the Queensland University of Technology (QUT) and the Translational Research Institute (TRI) in Brisbane, Queensland, Australia. During its due diligence, EpicentRx consistently came across a 2018 manuscript authored by Richard Gordon in the journal Science Translational Medicine entitled, “Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice.” The data from this manuscript was largely behind the multi-billion-dollar acquisition of Inflazome, a Queensland, Australia based biotech whose focus was on the inhibition of the NLRP3 inflammasome. The NLRP3 inflammasome is protein-based structure that kickstarts and maintains chronic inflammation, a common thread of many diseases including neurodegeneration.
To date, the preclinical collaboration with Dr. Gordon and the QUT, which officially kicked off over Zoom in 2020 during the COVID lockdown, has yielded three published manuscripts in high impact journals and two very sought-after grants with high dollar funding from the Michael J. Fox Foundation (MJFF)/Shake It Up Australia and Fight MND. Crucially, Dr. Gordon has unequivocally demonstrated that RRx-001/nibrozetone is a potent inhibitor of the NLRP3 inflammasome both in and out of the central nervous system (CNS) and that it is a definitive neurodegenerative disease modifier.
QUT to the End
On the expectation that Richard Gordon can do for EpicentRx what he did for Inflazome, the company hopes to open a satellite office in the QUT next to Dr. Gordon. Because Dr. Gordon plans to evaluate not only RRx-001/nibrozetone but also several of its metabolites in several relevant neurodegenerative disease models, a new tagline is under consideration: “test all you want, we’ll make more.”
