
We understand that Galera Therapeutics is currently going through a rough patch after the FDA’s refusal to approve their anti-mucositis agent, avasopasem manganese, and we sincerely wish them well. Avasopasem, a superoxide dismutase mimetic, is the latest anti-mucositis agent to come up short in Phase 3. Hopefully, however, that is not the end of the story or the road for avasopasem as the FDA has not rejected it outright.
Galera did not share what the exact issues with their Phase 3 trial called ROMAN were, so in lieu of an official explanation we can only offer our best guesses and assumptions. Our lead therapy, nibrozetone (RRx-001), in a soon-to-start Phase 2b trial called KEVLARx for SOM, trails closely behind avasopasem, so we have a vested interest to deduce what happened, the better to help with our own development of nibrozetone (RRx-001).
From our perspective, the first potential issue with the ROMAN clinical trial is that the magnitude of benefit for the primary endpoint was not impressively high and only just met statistical significance (p = 0.045) as avasopasem reduced SOM incidence by 16% compared to standard of care.
The second potential issue is one of convenience and compliance, which possibly relates to the low (ish) clinical benefit. Due to a short circulatory half-life of about 1 hour, avasopasem must be given 1 hour before the start of radiotherapy 5 days a week for 7 weeks: this amounts to a total of 35 times.
That is a lot of bother and hassle for cancer patients to deal with especially considering that radiation therapy centers are often located at some distance from the infusion centers where avasopasem is given. Add to that the tight 1-hour dosing window, which must be strictly adhered to, and it is reasonable to imagine that several patients, not all of whom have ready access to transportation, missed more than one of their 35 appointments or dropped out of the trial altogether. In a real-world situation where conditions are even less well controlled and less optimal than on a clinical trial, a higher number of missed appointments probably should be expected.
The third potential issue is that severe oral mucositis, as a complex process, which involves several pathogenic steps, may only optimally respond to agents that hit several targets relevant to the development of SOM. In the case of avasopasem, its one and only target is the superoxide anion, O2•–, which avasopasem dismutates to hydrogen peroxide, H2O2, as shown in Figure 1 below.
Figure 1: Avasopasem Mechanism of Action
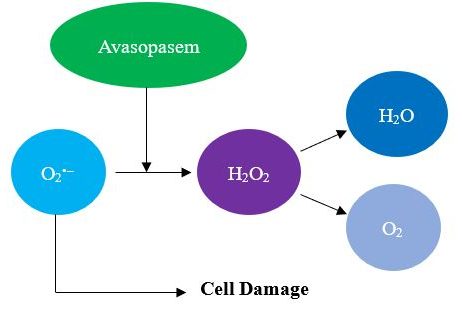
We do not mean to imply that superoxide anion is not a relevant target or that avasopasem, as a single targeted agent, is insufficiently active. To the contrary, the data suggests that superoxide is a relevant and important target, and since SOM often necessitates interruption of cancer therapy, which predisposes to recurrence, any reduction of its incidence, duration, or severity from avasopasem or another therapy is to be celebrated and welcomed with open arms.
The point we are trying to make here is only that a multitargeted agent, like nibrozetone (RRx-001), for example, which is also, by the way, much more convenient to administer, may possibly stand an even better chance to successfully tackle a multifactorial process like SOM.
However, in no way shape or form do we count avasopasem or Galera Therapeutics out, and we sincerely hope that Galera rebounds, with avasopasem as the first (or among the first) to find success in SOM.
After all, a rising tide lifts all boats.
